by Tate Hutchinson



Although the realm of particle physics may seem out of the scope of daily life, recent advancements have found an alternative use for the equipment used at the forefront of subatomic particle and dark matter study: a cutting-edge form of cancer treatment. The same technology that powers the 17-mile-long, $4.75 billion Large Hadron Collider in Switzerland can be used to treat cancer in a new, powerful, and less invasive method. And with millions worldwide facing new diagnoses each year, advancements in cancer treatment are crucial for saving lives in the future of medicine.
As opposed to the typical method of x-ray radiation, Proton Beam Therapy (PBT) is far more precise and controllable, posing far fewer risks to neighboring tissue. For many common cancers, those who opt for PBT receive chemotherapy, surgery, and the radiative treatment, the timeline and combination depending on the condition of the disease. PBT treatments are scheduled five days a week on average over several weeks until care is complete, although the daily therapy exposures only last a few minutes.
The Harlem Times spoke with Noah S Kalman, M.D. from Baptist Health South Florida, who specializes in radiation oncology and proton therapy. Growing up seeing his father work as a medical oncologist, he was inspired to enter medicine as a career to help people and care for patients. Now, they both work together at the Miami Cancer Institute, with his father, Leonard Kalman, M.D. serving as its Executive Deputy Director and Chief Medical Officer. MCI is at the cutting edge of proton therapy technology, suited with the newest equipment available.
As a radiation oncologist, Kalman feels inspired to get to know his patients and their families in order to best lead them through their treatment.

THE SCIENCE
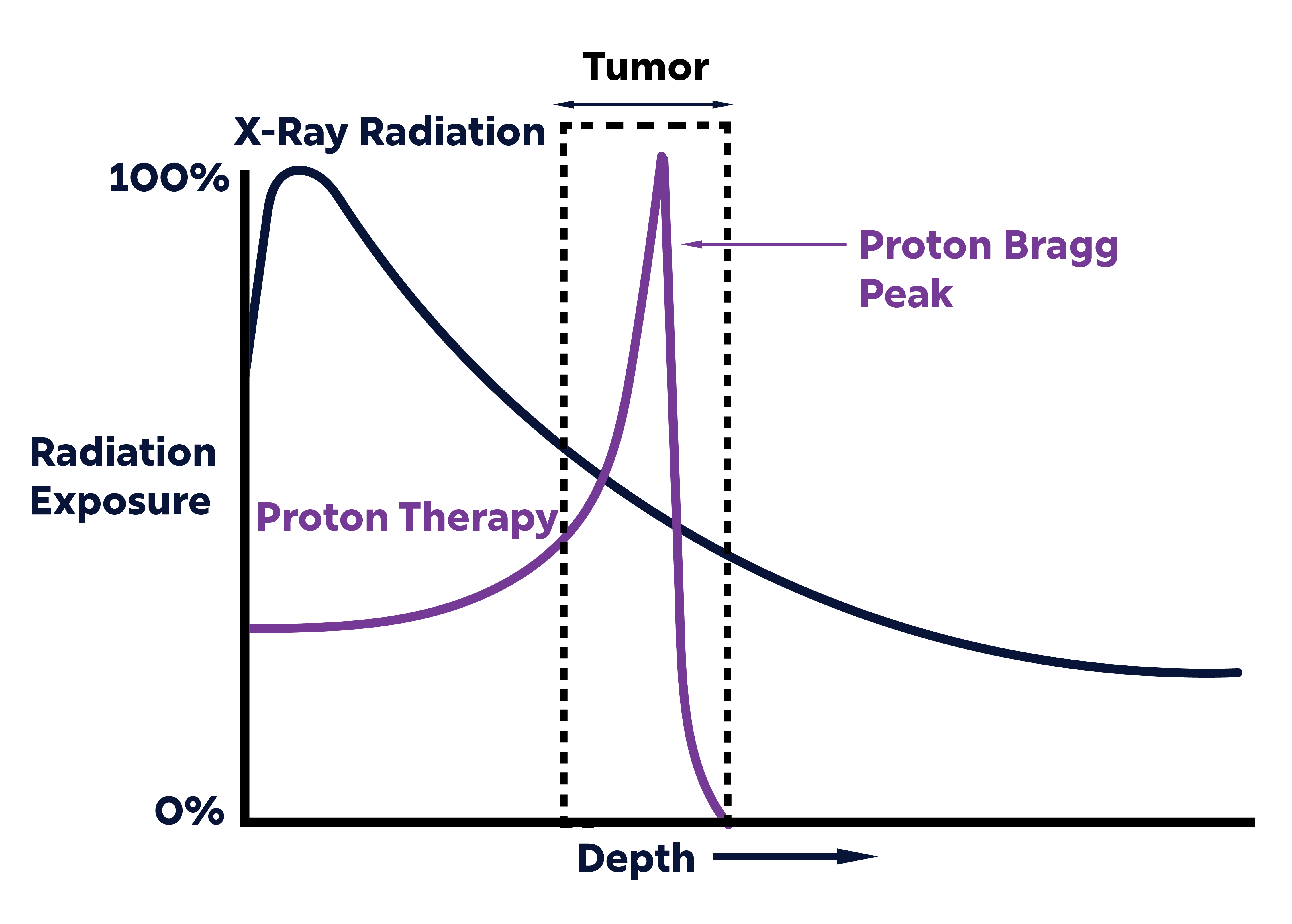
In proton therapy, a beam of protons is administered based on what is called the Bragg peak. As protons are accelerated, they amass kinetic energy that depletes rapidly as they pass through the body. When the energy is depleted, there is no further radiation deposited beyond that point. Medical scientists can control the velocity of the transmitted proton beam in order to deposit radiation at a specific depth, shrinking tumors by destroying DNA strands in cancer cells.
The science behind proton therapy more practically adapts what the world’s top physicists are researching at the Large Hadron Collider. Europe’s LHC was created to break new ground in physics research by generating new matter through particle collision at high speeds. Starting with a chain of linear (linac) particle preaccelerators and proton synchrotrons, larger and larger equipment speeds up particles until they reach maximum energy and collide in a massive ring of accelerators. And with an underwhelming number of major breakthroughs from the Large Hadron Collider, a subset of the study of particle acceleration physics has pivoted to medical research.
Clinical ion therapy centers are composed of large-scale accelerators, not too dissimilar from the ones used for research. Just like the LHC, medical proton beam facilities contain: (1) sources of ions such as protons, (2) linac accelerators to rapidly accelerate protons from standstill, and (3) an accelerator ring with superconducting magnets for protons to reach the necessary velocities to deliver a beam to the patient.
In medical use, however, the objective is not to create collisions and discover new particles, but instead to create a high-velocity beam that travels at the exact velocity needed to dose the tumor.
In addition to its aforementioned precision, PBT provides many benefits that improve upon the status quo of x-ray treatments. X-ray radiation emits a beam of high-energy photons (tens of cm across) targeted at the tumor site that administers the most radiation at the beam’s entry point, decreasing in damage as it goes fully through the body.
Since the radiation produces the most damage at the beam’s entry and continues beyond the tumor, it can lead to unnecessary damage and harmful side effects to additional tissue.
But proton beams (< 1 cm across), isolated at a targeted depth, are less invasive as they deposit less radiation at the entry point and no dose beyond the end of the beam. And especially as many undergo PBT concurrently with chemotherapy, minimizing the side effects of treatment is crucial for limiting unnecessary hospitalizations with complications of tissue damage.
As summarized by Dr. Kalman, “Proton therapy delivers radiation to our treatment target while reducing the amount of radiation received by healthy tissue. Proton therapy enters the body and then stops, delivering radiation to the target area with minimal exit dose. Proton therapy helps us treat tumors effectively while keeping the side effects of treatment as low as possible.”

Proton radiation therapy, with this increased precision, is particularly well-suited to treat tumors near tissues of other sensitive organs and those of close proximity to the skin surface. PBT treatment is optimal for cancers of the head, neck, and spine, pediatric cancers, as well as certain central organ tumors. Its high precision and limited invasiveness help protect the tissues that matter the most and limit side effects at an excellent rate.
However, due to its somewhat recent development, there are some challenges that could complicate its use worldwide. Although the radiation treatment itself is undetectable and does not cause pain, this form may cause extensive fatigue over time, limiting the energy one has to complete many daily activities.
And despite many leading scientists believing it to be a far safer method, there are few longitudinal studies so far to confirm this claim due to its recency. Additionally, proton therapy comes at a higher price tag than other alternatives, while its recent development has made insurance companies less likely to cover the gap.
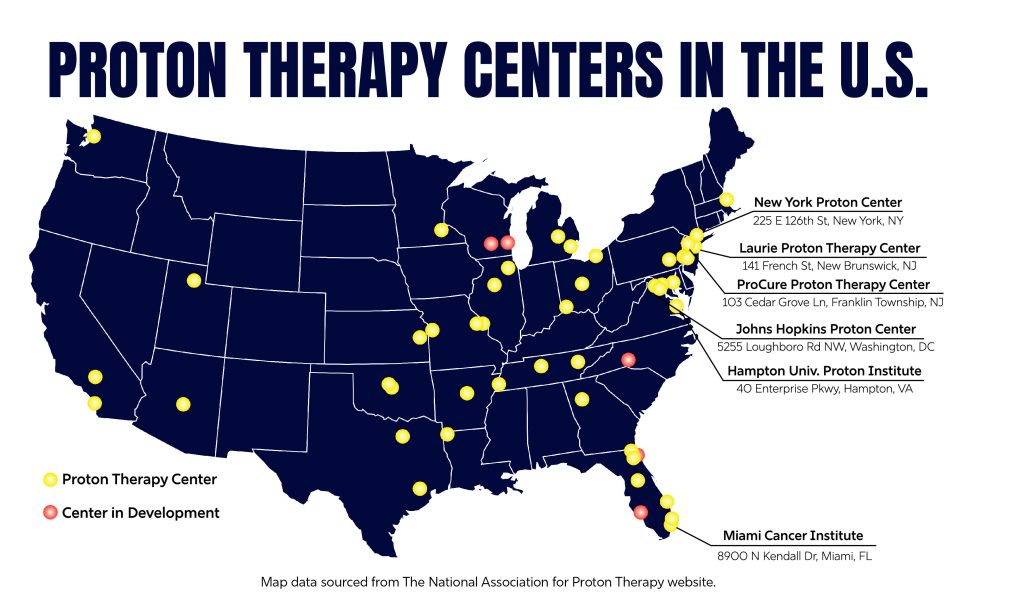
Despite these concerns, PBT is an encouraging treatment technology that works especially well for those most sensitive types: pediatric cancers and tumors close to radiosensitive tissue. There are 45 operating centers for proton therapy in the United States, including three in metropolitan NYC, and many more large medical centers are working on adding specialized equipment for this method that could save the lives of many and revolutionize the way cancer is treated.
In terms of where the future of cancer treatment is headed outside of proton therapy, Dr. Kalman suggests that meaures to predict the effects of a treatment plan can optimize oncology even further. As both traditional radiation and proton therapy technology continue to improve, understanding the biological effects of cancer treatment on different individuals is the largest advancement on the horizon.
“Developing tools to predict treatment response is the future of cancer treatment. We want to evaluate before and especially during treatment whether the treatment is working. Studies are ongoing to use imaging, blood tests, and other analyses during treatment to determine whether patients need additional or different treatments, or whether we can even stop treatments earlier than normal.”
The Harlem Times will continue to cover proton therapy and other cutting-edge treatments in upcoming editions. Stay tuned for more.